FDA Registered vs. FDA Cleared – What MedSpa Owners Must Know
FDA Registered vs. FDA Cleared: What MedSpa Owners Must Know Before Buying a Laser Hair Removal Machine
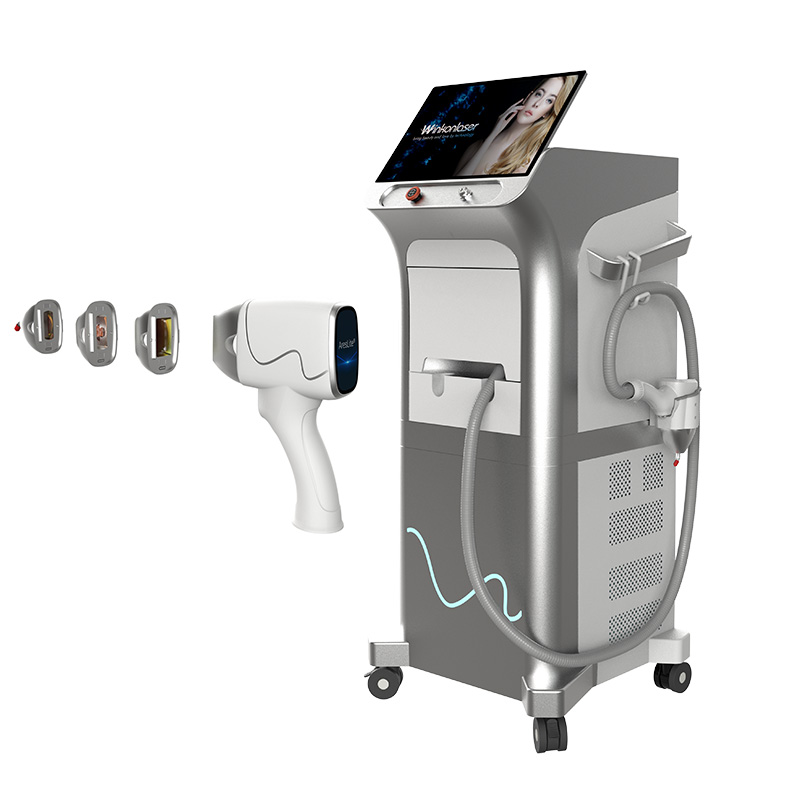
If you are in the market for a professional **diode laser hair removal machine**, you have likely encountered a flood of suppliers claiming to be “FDA Approved” or “FDA Certified.”
For a MedSpa owner or dermatologist, navigating these claims is not just about getting a good machine—it is about legal survival.
The truth is, **”FDA Approved” is a term rarely used for Class II medical devices** (like laser hair removal systems). Instead, the two terms you need to understand are **”FDA Registered”** and **”FDA 510(k) Cleared.”**
Confusing the two can lead to customs seizures, denied insurance claims, and even the loss of your medical license. In this guide, we break down the critical differences and explain why **Winkonlaser’s FDA 510(k) Clearance (K241860)** is your safeguard against these risks.
The Trap: What Does “FDA Registered” Actually Mean?
Many suppliers on B2B platforms will show you a certificate that says “FDA Establishment Registration.” It looks official, but here is the dirty secret:
“FDA Registration” does not mean the device has been tested for safety.
**What it is:** Establishment Registration simply means the factory has paid an annual fee to the FDA and listed their business address. It is like listing your business in a phone book.
**What it is NOT:** It does **not** mean the FDA has reviewed the laser machine itself. It does not prove the machine works, and it does not prove it is safe for human use.
**The Risk:** If you buy a laser that is *only* “FDA Registered” but lacks “510(k) Clearance,” you are technically importing an unapproved medical device.
The Gold Standard: What is “FDA 510(k) Cleared”?
For a laser hair removal machine to be legally marketed and used in the United States, it must have **510(k) Clearance**.
This is a rigorous review process where the manufacturer must submit comprehensive data to the FDA proving that their device is **”substantially equivalent”** to existing legally marketed devices in terms of safety and effectiveness.
**Safety Review:** The FDA analyzes clinical data, energy output stability, and safety mechanisms (like skin cooling and emergency stops).
**The “K” Number:** Every cleared device is assigned a unique “Premarket Notification Number,” which starts with the letter **K**.
**Without this “K number,” the device is not cleared for commercial distribution in the US.**

Why This Matters for Your Business ROI
Investing in a machine like the **Winkonlaser Ares Series** is not just about hair removal; it is about risk management. Here is why relying on a non-cleared device is a bad business decision:
Customs Seizures (CBP)
The US FDA works closely with Customs and Border Protection. If a shipment arrives labeled as a medical laser but lacks a valid 510(k) number linked to the specific manufacturer, customs will likely detain and destroy the machine. You will lose your entire investment with no recourse.
Malpractice Insurance
If a patient gets burned or injured (which can happen with unstable, non-cleared lasers), your malpractice insurance provider will first ask for the device’s FDA clearance status. If the machine was not legal to use, your claim will be denied, leaving you personally liable for damages.
State Board Licensing
Most State Boards of Cosmetology and Medicine require that all equipment used in a licensed facility be FDA-cleared. Using gray-market equipment can result in heavy fines or the revocation of your operating license.
Winkonlaser: Verification, Not Just Promises
At Winkonlaser, we believe in total transparency. We don’t just tell you we are compliant; we prove it.
The **Winkonlaser Ares Series (including AresLite and AresMix)** has successfully passed the FDA’s rigorous review process.
**Our FDA 510(k) Number:** **K241860**
**Product Code:** GEX (Laser surgical instrument for use in general and plastic surgery and in dermatology).
**Regulation Number:** 878.4810
Because we hold this clearance, our machines are recognized as safe and effective for permanent hair reduction. This allows you to market your treatments with confidence and ensures you are fully eligible for professional liability insurance.

How to Verify Our Clearance Yourself
We encourage every potential client to perform “Due Diligence.” Do not take a supplier’s word for it—check the official government database.
Go to the **FDA 510(k) Premarket Notification Database**.
In the search bar, enter our number: **K241860**.
You will see the official record for **Winkonlaser** and our device listing.

Conclusion: Don’t Gamble with Your Clinic’s Future
When choosing a supplier for your laser hair removal business, the price tag is important, but compliance is non-negotiable. Saving a few thousand dollars on a non-cleared “gray market” machine is not a saving—it is a liability waiting to happen.
Choose the **Winkonlaser Ares Series**. With our **FDA 510(k) Clearance (K241860)**, our **US Local Warehouse**, and our proprietary **Non-Crystal FAC Technology**, you are investing in a medical-grade asset that builds trust with your patients and protects your bottom line.
**Ready to upgrade your clinic with a fully compliant diode laser?**
[Contact our US Support Team] or [View the Ares Series Details Here].













Leave a Reply
You must be logged in to post a comment.